Medical Device Analytical Testing Outsourcing Market Size, Share, Growth Report 2032

The global market for medical device analytical testing outsourcing was estimated to be worth USD 5.55 billion in 2023 and is expected to grow to USD 11.28 billion by the end of 2032, according to a report released by Zion Market Research. Throughout the projected period, the market is anticipated to expand at a CAGR of 8.20%. The study examines the factors that will propel growth, impede it, and affect demand in the worldwide medical device analytical testing outsourcing market over the course of the projected year. It will also assist in navigating and investigating the prospects that present themselves in the Medical Device Analytical Testing Outsourcing Market sector.
✈👉Get a Free Sample: 🚀https://www.zionmarketresearch.com/sample/medical-device-analytical-testing-outsourcing-market
Introduction:
- Overview of Medical Device Analytical Testing Outsourcing:
Introduce the concept of outsourcing analytical testing services for medical devices. Analytical testing involves rigorous evaluation of medical devices to ensure their safety, performance, and compliance with regulatory standards. Testing services include material characterization, microbiological testing, mechanical testing, and chemical analysis, among others. - Market Importance:
Explain the growing importance of outsourcing these services to third-party organizations due to the increasing complexity of medical devices, stringent regulatory requirements, and the need for specialized expertise. Highlight how outsourcing helps companies reduce costs, streamline operations, and speed up product development.
Overview of the global market for outsourcing analytical testing for medical devices
The practice of focusing on third-party contracts for the supply chain management of medical device production, validation, packing, prototyping, product development, and verification in a controlled and sterile environment is known as medical device analytical testing outsourcing.

Growth Factors for the Global Medical Device Analytical Testing Outsourcing Market
The global need for outsourced medical device analytical testing is being driven by the rising incidence of chronic illnesses worldwide. The global market for medical device analytical testing outsourcing is expanding as a result of the government’s increasingly strict regulations regarding the quality certificate. Premarket approvals and 510(k) clearance are required for Class II devices; however, obtaining this clearance is a somewhat complicated process. As a result, these new developments have contributed to the expansion of the worldwide market for medical device analytical testing outsourcing.
Manufacturers of medical devices are using consultancy services to help them comprehend the paperwork and rules needed to get pre-market approvals. Since there is now less direct human contact during surgery thanks to the development of sophisticated instruments like surgical microscopes, surgical robots, neurosurgical devices, ophthalmic surgical devices, and many more, it is now required for medical devices to have quality certificates. The global market for medical device analytical testing outsourcing is expanding as a result of the rising demand for minimally invasive surgeries. To meet the necessary performance standards, manufacturers are turning to external medical device analytical testing services to ensure accuracy, precision, quality control, and routine maintenance.

Market Segmentation for Global Medical Device Analytical Testing Outsourcing
There are various ways to segment the global market for medical device analytical testing outsourcing, such by end-user, device type, therapeutic area, services, and region.
The market can be divided into hospitals and other end-user segments. The hospital industry has a hegemonic position over others because of its large budget and large patient base. It is possible to further divide the hospital segment into consumables and equipment. On the other hand, growth in ambulatory care, diagnostic centers, and specialty care facilities is anticipated during the projection period in other healthcare domains as well.
The market can be divided into recycled devices and other device kinds. Due to the high cost of therapeutic and single-user devices, the other section of the medical device analytical testing outsourcing market is dominant worldwide.
Therapeutic areas: Diabetes care, dental, endoscopy, medication delivery, general & plastic surgery, ophthalmology, IVD, orthopedics, diagnostic imaging, cardiology testing, and others can be used to segment the market. Due to the complexity of these devices and the need for highly skilled technical personnel, the cardiology testing sector holds the highest proportion of the global medical device analytical testing outsourcing market. Over the course of the projected period, it is also expected that the general and plastic surgery segment will rise significantly.
The market can be divided into segments based on services, such as material characterisation, bioburden testing, physical testing, sterility testing, and extractable and leachable.
✈👉Directly Purchase a copy of the report with TOC: 🚀https://www.zionmarketresearch.com/toc/medical-device-analytical-testing-outsourcing-market
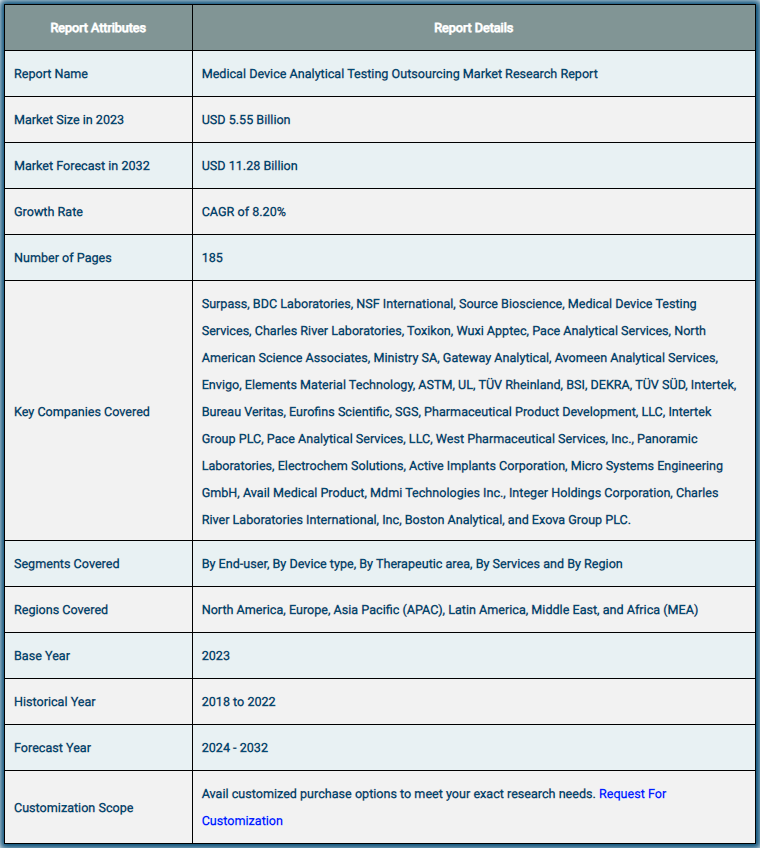
Market Report Scope: Medical Device Analytical Testing Outsourcing
Regional analysis of the global market for medical device analytical testing outsourcing
The region of Asia Pacific holds the highest market share in the global medical device analytical testing outsourcing industry, mostly because of government initiatives and improved healthcare infrastructure. Furthermore, during the projected period, the swift economic advancements in nations such as China and India are anticipated to considerably enhance the expansion of the regional market.
Due to the fast expansion of medical device manufacturing to meet the region’s soaring need for effective healthcare facilities, North America is also anticipated to develop significantly throughout the projection period. The area is renowned as the leading center for producing sophisticated, dependable, and expensive medical equipment.
Market Drivers:
- Stringent Regulatory Requirements:
The rising need for compliance with regulatory frameworks set by bodies such as the FDA (U.S.), EMA (Europe), and other international agencies is driving demand for outsourcing analytical testing services. - Increasing Complexity of Medical Devices:
As medical devices become more sophisticated (e.g., wearable technology, drug-device combinations, implants), they require advanced and specialized testing, often beyond the in-house capabilities of manufacturers. - Cost and Time Efficiency:
Outsourcing testing allows medical device companies to reduce overhead costs and shorten the time-to-market by leveraging external expertise and infrastructure without needing to build in-house labs or hire specialized teams. - Growing Demand for Medical Devices:
The aging global population, increased healthcare access, and rising prevalence of chronic diseases are boosting demand for medical devices, thereby driving the need for comprehensive testing services.
Market Restraints:
- Quality Control and Communication Issues:
Concerns about the quality control of outsourced services, especially in regions with less stringent oversight, and potential communication gaps between device manufacturers and service providers can be a challenge. - Data Security Concerns:
Given the sensitive nature of medical device development, maintaining the confidentiality of data while outsourcing can pose a risk. - Limited Flexibility:
Outsourcing can sometimes lead to a lack of flexibility in the testing process, with companies dependent on third-party timelines and workflows.
Market Segmentation:
- By Service Type:
- Material Characterization: Includes testing of polymers, metals, and ceramics used in medical devices to ensure biocompatibility and performance.
- Microbiological Testing: Testing for contamination and sterilization validation, critical for devices like surgical instruments and implants.
- Mechanical Testing: Evaluates the mechanical properties of devices, including strength, durability, and fatigue resistance.
- Chemical Testing: Ensures that chemical interactions within devices meet safety and performance standards.
- Analyze the market share of each testing service and highlight which segments are expected to grow due to evolving regulatory demands and technological advancements.
- By Device Type:
- Class I Medical Devices: Low-risk devices such as bandages and surgical instruments.
- Class II Medical Devices: Moderate-risk devices, including infusion pumps and diagnostic equipment.
- Class III Medical Devices: High-risk devices like pacemakers, implantable defibrillators, and drug-device combinations. These devices require rigorous testing and are a key driver of market growth.
- By End-User:
- Medical Device Manufacturers: The primary users of outsourcing services.
- Contract Research Organizations (CROs): Often outsource testing services as part of their larger clinical and regulatory support for device manufacturers.
- Hospitals and Research Institutes: Limited end-users, typically for specialized device trials or custom device development.
Regional Insights:
- North America:
The largest market due to strict FDA regulations, a large base of medical device manufacturers, and strong infrastructure for outsourcing services. - Europe:
Significant growth is expected due to the stringent Medical Device Regulation (MDR) and In-Vitro Diagnostic Regulation (IVDR) that require higher levels of device testing and compliance. - Asia-Pacific:
Emerging as a lucrative market, driven by lower testing costs, a growing number of medical device manufacturers, and increasing investments in healthcare infrastructure, particularly in countries like China, Japan, and India. - Rest of the World (RoW):
Discuss the growing market potential in Latin America, the Middle East, and Africa as healthcare systems evolve and demand for medical devices increases.
Competitive Landscape:
- Key Players:
Discuss major companies providing outsourcing services, such as Charles River Laboratories, Eurofins Scientific, Intertek Group plc, SGS SA, and WuXi AppTec. Highlight their global presence, service offerings, and strategic initiatives such as mergers, acquisitions, or collaborations. - Service Expansion and Technological Advancements:
Focus on how companies are expanding their testing capabilities to meet the growing demand for more specialized and advanced analytical services, including biologics and combination devices.
Future Outlook and Trends:
- Growth of Combination Products:
With the rise of drug-device combinations and biologics, testing requirements are becoming more complex, opening new avenues for specialized outsourcing services. - AI and Digital Health Integration:
Emerging technologies like artificial intelligence (AI) and machine learning are being used to optimize analytical testing and enhance the accuracy of results, driving future growth. - Globalization of Medical Device Markets:
As medical device markets expand globally, especially in emerging regions, the demand for outsourced testing services will rise to meet varying international regulatory standards. - Sustainability and Green Testing:
Explore the trend towards more environmentally friendly and sustainable testing processes, with companies looking to reduce the environmental impact of their operations.
Conclusion:
Summarize the key factors driving the growth of the medical device analytical testing outsourcing market, including regulatory pressures, increasing device complexity, and cost efficiency. Highlight future opportunities, such as growth in emerging markets and the role of advanced technologies in shaping the future of outsourcing services.
✈👉Enquiry for buying: 🚀https://www.zionmarketresearch.com/inquiry/medical-device-analytical-testing-outsourcing-market
get to know better
Label Printing Machines Market
Contact Us:
Zion Market Research212
USA/Canada Toll Free: 1 (855) 465–4651
Newark: 1 (302) 444–016611\
Web: https://www.zionmarketresearch.com/
Blog: https://zmrblog.com/
%20Market%20main%20jpg.png)
Comments
Post a Comment